Surface Plasmon Resonance
Room 612
Contact: K. Skowronek
Access: collaboration/contracted research, experienced users (internal)

Biacore S200 (GE Healthcare), 20General description
This technique is used for detailed kinetic characterization of bi-molecular interactions. One of interacting partners (interactants) is immobilized on sensor surface (ligand) while the other one (analyte) is injected with a buffer. By measuring SPR angle, changes of refractive index in ~200 nm layer on the sensor surface are recorded in a real time (sensorgram). This technique does not require labelling of interactants nor impose any limitations on buffer. Series of sensorgrams obtained at different analyte concentrations are used in global fitting to obtain koff and kon kinetic constants of an interaction. The method is relatively fast (one injection usually takes 10-20 minutes) and consumes minute amounts of interactants (~0.5 ml of ligand and analyte solutions at concentrations ~ 5 x expected KD).
Applications
- Kinetic characterization of molecular interaction. Especially useful for comparing multiple analytes (small molecules, protein variants) or buffer impact
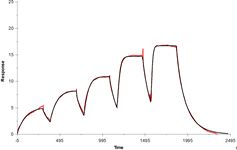
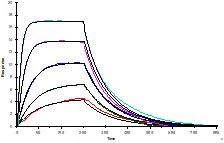
- Affinity measurement. Only equilibrium KD is measured but such assay is far more robust than full kinetic characterization.
- Inhibitor ranking/characterization. Influence of third component (one or multiple) on bi-molecular interaction can be studied.
- Library screening. Medium throughput method for screening best ligand-binder in library of up to 394 potential interactants in a single unattended experiment.
- Antibody characterization (ranking, binning, mapping)
- Thermodynamic analysis. Series of measurements is performed at different temperatures and van ‘t Hoff plot and is used to estimate interaction enthalpy and entropy
Sample requirements
Ligand: Monodispersed and homogeneous or selectively “capturable” (selective capture of single His-tagged ligand from heterogeneous mixture is in principle possible); lack of components interfering with capture technology (histidine-rich contaminants for Ni-NTA sensors; free biotin for streptavidin sensors; primary amine containing buffer components for NHS-EDC chemical cross-linking)
Analyte: Stable in assay buffer (no aggregation!); known concentration; equilibrated in an assay buffer
Further reading
- https://www.sprpages.nl/
- https://cdn.gelifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=16477
- Handbook of Surface Plasmon Resonance 2nd Edition, Richard B M Schasfoort (Editor), Royal Society of Chemistry 2017. ISBN-13: 978-1782627302. ISBN-10: 1782627308
- Z. Gorodkiewicz E, Lukaszewski Z. Recent Progress in Surface Plasmon Resonance Biosensors (2016 to Mid-2018). Biosensors (Basel). 2018, 8(4). pii: E132. doi: 10.3390/bios8040132. PubMed PMID: 30558384.
- Nguyen HH, et al. Surface plasmon resonance: a versatile technique for biosensor applications. Sensors (Basel). 2015, 15(5):10481-510. doi: 10.3390/s150510481. PubMed PMID: 25951336.
- Patching SG. Surface plasmon resonance spectroscopy for characterization of membrane protein-ligand interactions and its potential for drug discovery. Biochim Biophys Acta. 2014, 1838(1 Pt A):43-55. doi: 10.1016/j.bbamem.2013.04.028. PubMed PMID: 23665295.