SEC-MALS
Size Exclusion Chromatography – Multi-Angle Light Scattering
Room 607A
Contact: Krzysztof Skowronek
Access: collaboration/contracted research, experienced users

Components:
- Binary HPLC Pump 1525 (Waters)
- Inline Prep Degasser (Waters)
- Manual Injection module Flexinject (100 µl and 5 ml loops) (Waters)
- UV/VIS Detector 2489 (Waters)
- Column oven (for up to 30 cm columns) (Waters)
- Dawn 8+, 8 angles MALS detector (Wyatt)
- Optilab T-rEX refractive index detector (Wyatt)
- Fraction Collector III (Waters)
General description
This instrument allows calibration-independent measurement of molecular mass in each fraction of SEC. UV/VIS, MALS and refractive index (RI) detectors are in-line with outflow from HPLC system. UV/VIS and RI detectors are used for independent concentration quantification while signal from 8 MALS detectors provides information on light scattering at different angles. MALS data are analysed by selected mathematical formula (by default it is Zimm eqution) to obtain calibration-independent time-resolved molecular mass measurement.
NOTE: In contrast to analytical ultracentrifugation[L] MALS directly measures radius of gyration (not hydrodynamic radius). SEC fractions can be collected by fraction collector for further analysis. Currently we offer two SEC columns:
- BioSuite 450 Å (8 µm, 7.8 x 300 mm) with resolution range 20 kDa – 1 MDa (for globular proteins)
- XBridge Protein BEH SEC 200 Å (3.5 µm, 7.8 x 300 mm) with resolution range 10 kDa – 450 kDa (for globular proteins)
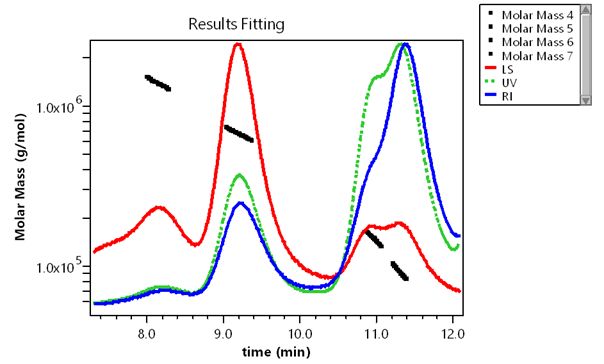
SEC-MALS of Waters molecular weight standards resolved on BioSuite 450 column. Peaks: 1 – dimeric thyroglobulin (1.4 MDa), 2 – monomeric thyroglobulin (660 kDa), 3 – IgG (150 kDa), 4 – BSA (66.4 kDa)
Applications
- Measurement of molecular mass of various biological macromolecules (polysaccharides [using RI detector], proteins, nucleic acids and their complexes)
- Estimating homogeneity of macromolecule preparation
- Estimating monodispersity of macromolecules in each fraction
- Assisting in the last step of macromolecular complex purification (using fraction collector)
- Determination of stoichiometry of macromolecular complexes
Sample requirements
At least 120 µl at concentration adequate for proper estimation of concentration from relative RI (at least 1-2 mg/ml) equilibrated in chromatography buffer. Sample should be filtered through 0.2 µm filter.
Further reading
1. https://www.wyatt.com/library/theory/multi-angle-light-scattering-theory.html
2. Mogridge J. Using light scattering to determine the stoichiometry of protein complexes. Methods Mol Biol. 2015;1278:233-8. doi: 10.1007/978-1-4939-2425-7_14. PubMed PMID: 25859953.